Although more than a year past since COVID-19 was defined, there is no specific treatment yet. In this study, we aimed to evaluate the efficacy of the regimen with Favipiravir (FPV) and determine if the timing of FPV addition offers any improvement.
Introduction
This study aims to evaluate changes in hematological parameters after the follow-up of patients who received treatment with favipiravir due to COVID-19 infections. we aimed to evaluate the efficacy of the regimen with FPV and to find out if the timing of FPV addition offers any improvement. These findings will help enlighten clinicians for the clinical treatment of SARS-CoV-2 infection.
METHODS
Sixty-two cases receiving favipiravir treatment for at least five days due to COVID-19 infection were evaluated retrospectively. Parameters including age, gender, nasopharyngeal swab positivity, and chronic diseases were analyzed. Hematologic parameters were analyzed before and after the treatment.
RESULTS
The mean age of the patients receiving treatment with favipiravir was 63.7±12.3 years.. The most common comorbid conditions detected in patients were hypertension in 25 cases (40.3%) and diabetes in 16 cases (25.8%). In the statistical analysis of the hematological parameters before and after treatment with favipiravir, WBC, PT-PTT-INR levels were found to be unaffected; the mean RBC was found to have decreased from 4.33 ± 0.58 M/uL to 4.16 ± 0.54 M/uL (p:0.003); the median hemoglobin level was found to have decreased from 12.3 g/dl to 11.9 g/dl (p:0.041); the hematocrit level decreased from 38.1% ± 4.8 to 36.9% ± 4.2 (p:0.026)
CONCLUSION
We concluded that the pathological effect of treatment with favipiravir on the hematologic system was the suppression in the erythrocyte series, and there were no adverse effects in other hematologic parameters.
Know about favipiravir
Favipiravir is an antiviral used to manage influenza, and that has the potential to target other viral infections.
A favipiravir is structurally 6-fluoro-3hydroxy-2pyrazenecarboxamide and a broad spectrum antiviral drug showing in vitro activity against RNA viruses including influenza virus, respiratory syncytial virus and measles virus
Pharmacodynamics and Mechanism of Action
The mechanism of action of Favipiravir is special as other marketed influenza drugs either inhibit entry or release of the virus . For replication of RNA viruses RNA dependent RNA polymerase (RdRp) is needed as it controls the rate of replication and mutation of the virus to adapt within the host surroundings.22Favipiravir is a prodrug which enters the infected cells through endocytosis and it is transformed to active metabolites favipiravirribofuranosyl triphosphate ( Favipiravir-RTP) through phosphorylation and phosphoribosylation.It binds to and selectively blocks RdRp which in turn prevents viral transcription and replication.27 The disruption in viral RNA replication leads to increase number and frequency of translation mutations which replaces Guanine (G) by Adenine(A) and Cytosine (C) by Thymine(T) or Uracil (U) , thereby producing devastating mutagenesis in RNA viruses.
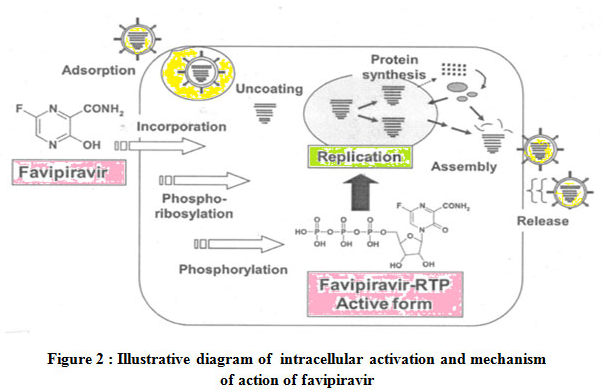
Illustrative diagram of intracellular activation and mechanism of action of favipiravir is shown in Figure 2.
Dose
The recommended dosage of favipiravir for adults is 1800 mg orally twice daily on 1st day followed by 800 mg orally twice daily, up to maximum of 14 days.
FAVIPIRAVIR SIDE EFFECTS
Some common side effects of the Fabiflu are swelling all over the body, muscle pain, asthma attack, gastrointestinal upset, miscarriages, hay fever, and allergic dermatitis.
The use of favipiravir in pregnancy is restricted as it may cause fetal damage.
WARNINGS AND PRECAUTIONS
- Favipiravir mainly causes early embryonic death when studied in animals. Recommendation of this medication should be avoided to pregnant women.
- The most effective contraceptive method should be taken during treatment. If pregnancy occurs the treatment should immediately be stopped.
- Avigan is not given in case of bacterial infection.
- Favipiravir is not recommended for children.
- This drug should not be consumed if the patient has any history of hypersensitivity with any ingredients of the product.
- The probability of distribution of Favipiravir in sperm is high. During the administration of this medication to male patients. Fully elaborate the risk and also instruct for avoiding the sexual intercourse during and for seven days and after the end of the treatment.
Brand names
Favipiravir is sold under the brand names





Write a comment
You must be logged in to post a comment.